How to get and keep a basic pH
So, first post about my passion for aquarium fish.
I talk about it much more to please myself than to try to share any science, Youtube is full of geniuses in all fields, Cichlids of the great African lakes included.
You are warned :)
Well, just to disgust 99% of people, let's start talking about aquarium science by talking about pH.
Why talk about chemistry instead of just talking about fish?
Because the fish I chose come from Lake Tanganyika, which has a very particular pH, because it is very basic.
If you remember your chemistry classes, the pH varies between 0 (very acidic), 7 (neutral) and 14 (very basic).
Moreover, this pH is not linear (that would be too easy): for example, if you go from 7 to 8 the pH is 10 times more basic.
Basically in freshwater aquariums, there are two extremes: the Amazon area whose water is quite acidic (<7) and the African great lakes whose water is strongly basic (>8).
So how can I get water with a pH around 8.5 when when the one coming out of my tap it is at 7?
Two solutions:
- Add baking soda (30 grams per 100 liters) but water changes will modify this proportion so one must constantly reintroduce some of it, not practical.
- Put limestone rocks and substrate that will slowly dissolve and raise the pH to the desired value. Advantage ? Slower process (therefore not violent for fish that don't like sudden changes) and above all almost insensitive to continuous water changes since the dissolution is slow and constant.
I chose the second solution for its obvious advantages by placing in my tank a substrate dedicated to this type of biotope: sand from the Dolomites (it's white, that's what I wanted) and some limestone rocks (they are found in profusion everywhere, mine come from Provence).
With these two ingredients my pH has gently increased (in three months) from 7 to 8.6 and stabilized itself:
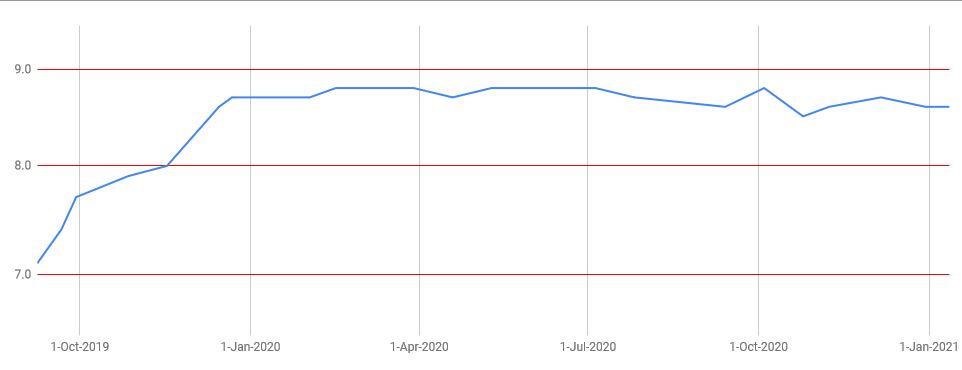
But how do you measure this pH?
It's a bit more complex than measuring temperature :)
In the beginning, I bet it all on the JBL ProScan.
Sexy (with the results on my smartphone), fast (2 minutes), it had everything to please.
Yes but ... it's not reliable. The values are very fluctuating: two tests done in a row can give pH variations between 7.5 and 8.5 (remember, a pH unit is a factor of 10 on acidity). Conclusion, I don't use it anymore.
So I bought an electronic Ph-meter (sold mainly for swimming pools).
It's not expensive, but the problem is that it often needs to be calibrated, and I don't want to spend my time buying the buffer powder needed for this action.
So? I searched and found the pH of the main mineral waters sold in bottles.
In France, I buy from time to time some Vittel (one of the most basic to get closer to the target pH range) whose pH is constant at 7.8, and I recalibrate my tester in a glass of Vittel ... before drinking it.
This way I hydrate myself, my pH is worthy of the banks of Tanganyika, and I calibrate for cheap.
I think we're done with the subject, next time, I'll talk about automatic water changes. Perhaps.
Have a great! as Ryan would say.
(no, it has nothing to do with fish, but I do what I want).
